
India has long been the focus of the world for clinical research particularly since 2005. The market has been always every investors’ focus of attention on account of India’s massive population, large patient pool from various races, cost arbitrage, wide spectrum of diseases and availability of English speaking technical expertise in rising numbers. The Indian …
Continue reading “India clinical research market: Rebounding from post-2011 slump”
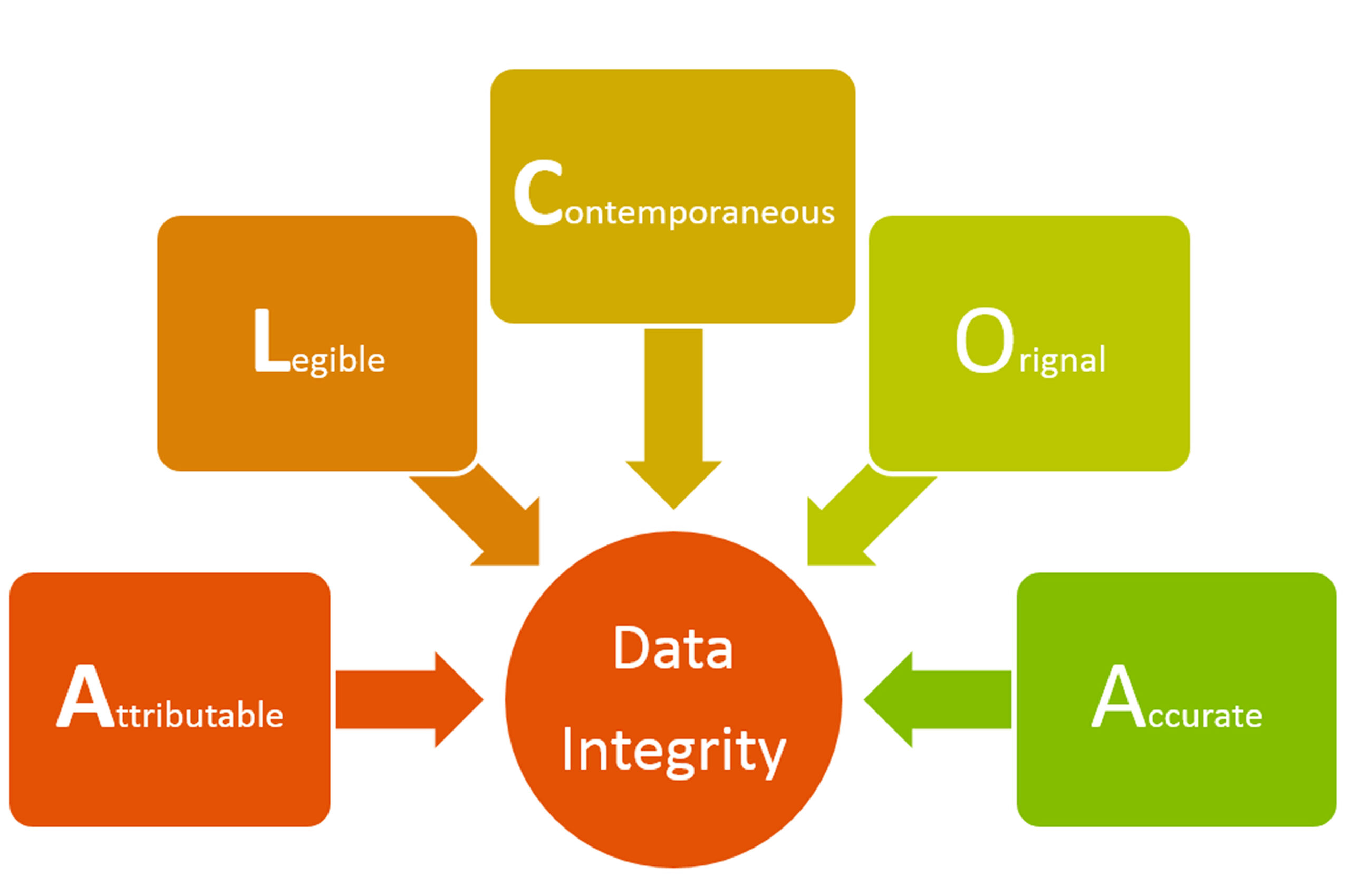
US FDA import warning due to data integrity problems IPCA Laboratories’ Ratlam unit received an import alert from the US drug regulator on 22 January 2015 for not complying with good manufacturing practices requirements of the US FDA. IPCA Laboratories is a leading manufacturer of pharmaceutical medicines producing greater than 350 formulations and 80 APIs …
Continue reading “US FDA import warning due to data integrity problems”
