US FDA import warning due to data integrity problems
IPCA Laboratories’ Ratlam unit received an import alert from the US drug regulator on 22 January 2015 for not complying with good manufacturing practices requirements of the US FDA. IPCA Laboratories is a leading manufacturer of pharmaceutical medicines producing greater than 350 formulations and 80 APIs for various therapeutic segments. The title of the import alert given by the US FDA to IPCA Laboratories was known as ‘Detention without physical examination of drugs from forms who have not met the GMPs’ popularly known as DWPE import alert. This is almost always followed by a Warning Letter indicating the extent of the problems at the facility.
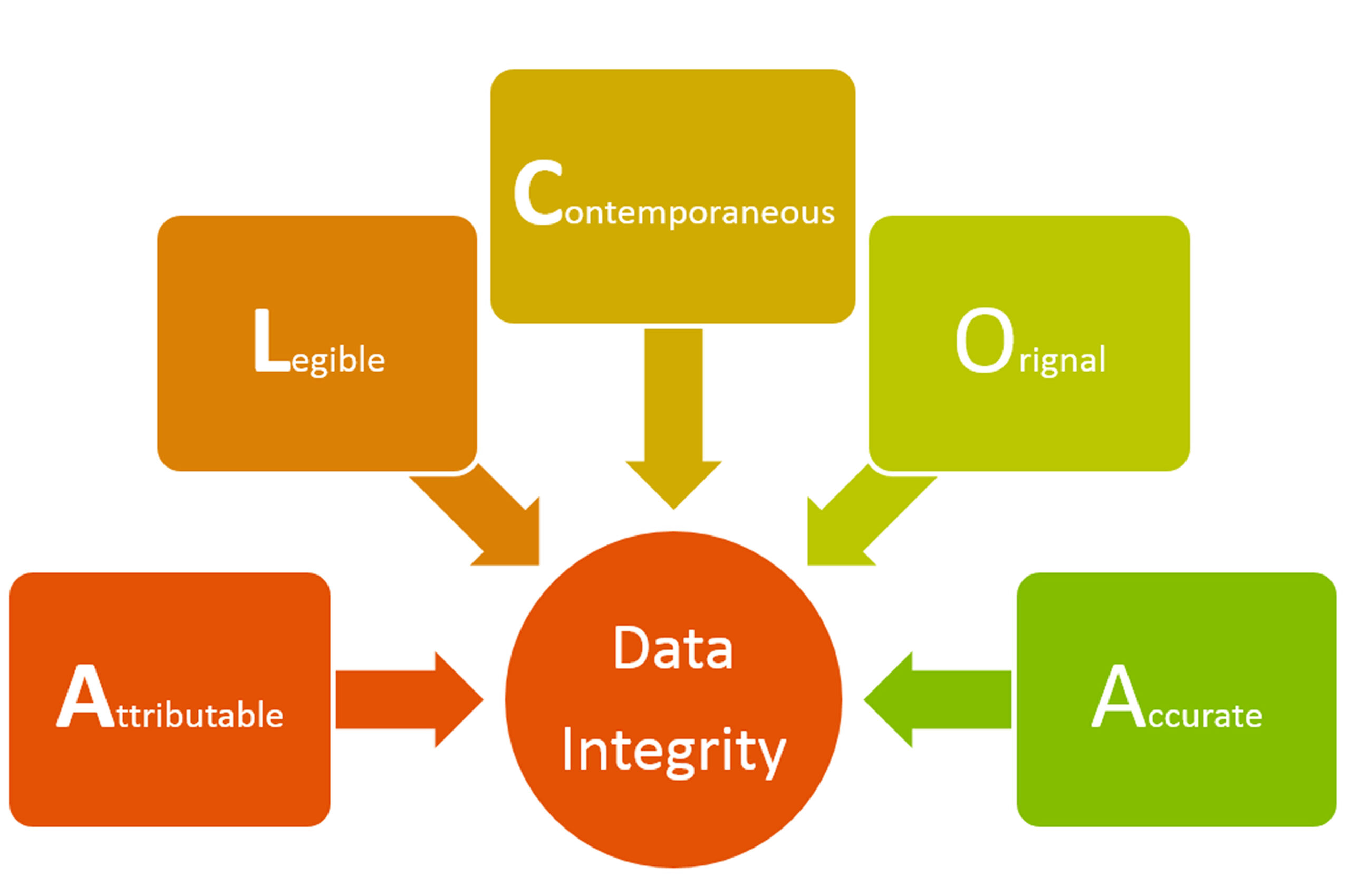
IPCA is not the only Indian pharmaceutical manufacturer to face the wrath of the US FDA. At least 13 Indian firms have been issued import alerts and subsequent warning letters since May 2013. Most the warning letters have been issued because of the problems of data integrity at pharmaceutical manufacturing facility and US FDA has cited irregularities in the companies’ data integrity practices.
Analysis of US FDA import alerts by country
India and China pharmaceutical companies feature among the maximum number of import alerts for good manufacturing practices. Since 2009, 160 countries have been issued import alerts for violation of good manufacturing practices. The top 30 countries in import alerts contribute to 58 percent of the total import alerts. India and China top the list of import alerts in the USFDA scanner.